Weight Loss – Cataplerosis, Anaplerosis and PEPCK
Ever wondered what happens to protein and amino acids in the body apart from muscle repair and building?
OK, maybe not, but I’m going to tell you anyway…
This article is a bit of a geek-out and only my curiosity as to the further complexity of the mitochondria in the presence of a ketogenic diet, intermittent fasting, or exercising in a fasted state.
Overall Health Benefits of the Ketogenic Diet
But let’s start off with some of the basics:
-
“FED” STATE
The “fed” state occurs right after eating.
This is when insulin will be high and glucagon will be low. After protein is digested inside the stomach it moves to the small intestine where it triggers the release of incretins (gut hormones) such as GLP-1 and GIP which then signals for insulin to be released in preparation for the anticipated energy that’s coming its way.
Think about it. The stomach is essentially warning the rest of the body that there’s incoming nutrients which allows all the other organs such as the pancreas and liver to get ready before the nutrients even get absorbed.
From the breakdown of proteins, amino acids are formed that is then transported to the liver where it will either go to:
- Protein synthesis (making building blocks), or
- Energy production (breaking it down for energy)
Energy Production
In the case of energy production, protein or amino acids can be converted into either:
- Glucose
- Fatty acids
Glucose
Glucose is stored as glycogen in the liver.
The precursors for glucose are:
- Pyruvate, and
- Oxaloacetate – this is usually in balance with other intermediaries of the krebs cycle.
Glucogenic amino acids will feed into this part of energy metabolism.
Gluconeogenic amino acids are intermediary amino acids that can enter the krebs cycle via anaplerosis to be converted into glucose molecules, in short, proteins that can be turned into sugar when the body needs glucose via a process called gluconeogenesis. These amino acids can also be converted directly into pyruvate to enter glycolysis. This process takes place in the liver usually in response to starvation or fasting. The majority of amino acids are gluconeogenic and include:
- Glycine
- Serine
- Valine
- Histidine
- Arginine
- Cysteine
- Proline
- Alanine
- Glutamate
- Glutamine
- Aspartate
- Asparagine
- Methionine
Fatty acids
Fatty acids are stored as tri-acyl glycerides (TAG) in the fat cells (adipose tissue).
The main precursor for fatty acids is acetyl CoA. Acetyl CoA is usually in balance with Acetoacetyl CoA.
Ketogenic amino acids will feed into this part of energy metabolism.
Ketogenic amino acids are amino acids that can be converted directly into acetyl-CoA or acetoacetyl CoA to become fatty acids (TAG’s), but cannot enter the krebs cycle, so is taken out of the liver to be converted into ketone bodies. These include:
- Leucine
- Lysine
A small portion of amino acids can be classified as both gluconeogenic AND ketogenic:
- Phenylalanine
- Iosleucine
- Threonine
- Tyrosine
- Tryptophan
Protein Synthesis
If enough fuel is on board for energy production, then amino acids will move to other cells in the body like the muscle where it is used as building material to repair cells and aid in the growth of cells, ie muscle building.
-
“FASTED” STATE
During fasting, starvation, or energy deprivation, fuel has to be sourced from other areas to be processed by the liver to suitable fuel sources for the rest of the organs to function, the main being:
- Fats
- Amino acids
Fats
Fats are broken down into fatty acids that is then transported to the liver where it undergoes oxidation to provide ATP/energy for the liver to make glucose or ketones (depending on the length of time)
Ketones
The production of ketone bodies is a way for the body to preserve or spare muscle and amino acids from being the primary fuel source in order to keep energy production sustainable.
Amino acids
As previously mentioned, amino acids can be converted in the liver into glucose (glycogen) of fatty acids (TAG’s) for energy. Some of the amino acids can become intermediaries of the krebs cycle and contribute to some ATP production via krebs, but amino acids only contribute about 10-15% of total energy production, so the majority or energy will still come from fatty acids. Where the amino acids are important though is that they provide the carbon-backbone (α-keto acid) for glucose synthesis.
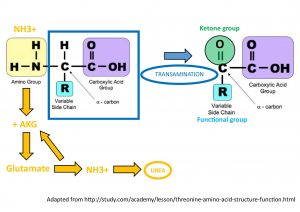
So let’s look at the basic structure of an amino acid:
All amino acids have a central carbon molecule to which you have attached an amino group, carboxylic acid group, hydrogen and variable side chain. The side chain is what will differ between amino acids and make them unique. The nitrogen in the amine group is what makes the breakdown of amino acids different to that of glucose or fatty acids. In order to separate the carbon-backbone (which we need for glucose or fatty acid synthesis and thus energy) from the nitrogen-amine group, the amino acid molecule has to undergo a process called TRANSAMINATION. The amine group is then transferred to another molecule called α-ketoglutarate (intermediary of krebs) to become glutamate.
Glutamate then goes to the liver where liver enzymes enables the glutamate to donate this amine group to ammonia where it enters the urea cycle to be excreted by the kidneys through the urine and so frees up the carbon-backbone which is now called an α-keto acid. This α-keto acid now can contribute to the energy metabolic pathways.
So how does the mitochondria feature in all of this?
MITOCHONDRIA
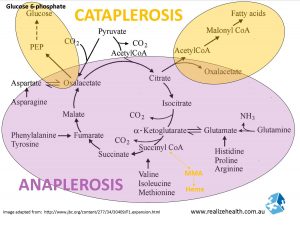
Cataplerosis & anaplerosis are 2 biochemical pathways in the krebs cycle that help to break down carbohydrates and fats for energy. The krebs cycle involves the constant recycling of 4 carbon molecules in these energy creating processes. 2 carbons attach to the 4 carbons to make a 6 carbon molecule. The 2 added carbons is removed to provide energy and the whole cycle starts again.
During this process essential amino acids enter the krebs cycle as intermediaries (as mentioned above glucose to be in balance with oxaloacetate) and spits out non-essential amino acids, if you can imagine the krebs cycle spinning like a wheel in a clock-wise direction.
These pathways become more important during ketogenesis when carbohydrate intake and cellular pyruvate concentrations are low, because there isn’t a lot of substrate (glucose) going in through PDH (Pyruvate Dehydrogenase) to enter the krebs cycle at the top of the diagram.
-
ANAPLEROSIS
Anaplerosis is the ‘breaking down’ process and pulls amino acids into the krebs cycle, the two main ones being glutamate (converted to alpha-ketoglutarate) and aspartate (converted to oxaloacetate) and burns them for energy leaving CO2 and water. Essential amino acids that are able to enter here, which includes BCAA’s (branch chain amino acids), are converted to non-essential amino acids and glucose. Oxaloacetate is the main component that keeps the krebs cycle going and everything that occurs – fat, carb and protein breakdown – is to make oxaloacetate.
As mentioned before, it essentially involves the binding of a 2-carbon group (Acetyl CoA) to a 4-carbon group (oxalacetate) to form a 6 carbon-group (citrate) in the process. Citrate can then be broken up again as part of the CATAPLEROSIS pathway to form fatty acids and keep oxalacetate circulating in the krebs cycle.
The following is just my thoughts:
What happens when we supplement citrates?
This includes citrate minerals. I theorize that it would increase the production of fatty acids and possibly oxalacetate that keeps the cycle going. Maybe it will also inhibit acetyl CoA production and cause pyruvate to build up. Pyruvate will then be directly converted to oxalacetate, so maybe we see an increase in oxalacetate and intermediaries such as fumarate and succinate. However, in the absence of oxygen (yeast infections, exercise) pyruvate will just enter lactate production.
As long as the Krebs cycle is allowed to flow freely, it most likely won’t be an issue, but mitochondrial blocks or inhibitions may be one reason why some people react poorly to citrate.
-
CATAPLEROSIS
Cataplerosis is the ‘building’ of new materials. When amino acids are broken down for energy 4- and 5-carbon intermediaries (such as oxaloacetate and alpha-ketoglutarate) are formed in the krebs cycle. These intermediaries cannot be fully oxidized so they have to be removed or ‘spat out’ from the Krebs cycle through cataplerosis and then pushed into other pathways such as PEPCK, malonyl CoA and gluconeogenesis where they are used as the building blocks for making sugar, fat, or cellular growth.
PEPCK (P-enolpyruvate carboxykinase)
PEPCK is a gene encoding for phosphoenolpyruvate carboxykinase responsible for the first step in liver gluconeogenesis (glucose being produced in the liver from glycogen or amino acids). Glucagon, corticosteroids, stress and adrenalin will stimulate PEPCK expression whereas eating and insulin release will inhibit PEPCK. As mentioned, oxaloacetate is a 4-carbon intermediary molecule produced in the krebs cycle and metabolizes PEPCK to PEP. PEP goes through gluconeogenesis to become a full glucose molecule.
PEPCK expressed in fat cells will convert free fatty acids into triglycerides. So, PEPCK is responsible for making more glucose and triglycerides in the body – not a good combination.
Insulin regulates PEPCK expression. When blood glucose levels are high, insulin is secreted in order to remove the blood glucose. Because you don’t need to produce any more glucose, insulin will suppresses PEPCK secretion.
Metabolic Syndrome and Fatty Liver
But in diabetics with either no insulin or those who have insulin resistance, this doesn’t happen. PEPCK is not down-regulated and keeps on producing glucose which is why we see high fasting and non-fasting glucose levels in diabetics.
PEPCK will also be upregulated in a ketogenic diet. In the abscence of sugar and carbs from the diet, and insulin secretion, PEPCK will be more active in breaking down fats into triglycerides to be burned for fuel.
References:
PEPCK and type II diabetes (non-insulin-dependent diabetes mellitus)
Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation.