Have You Been Told That You Need To Eat Less Fat To Lose Weight Or To Improve Type 2 Diabetes?
What if I told you that dietary fat may not be the problem.
What if I told you that you should look at your carbohydrate and sugar intake instead, as well as the amount of exercise you are or are not participating in.
In very simple terms, if you eat a lot of sugar, carbohydrates, grains or starches, and you don’t exercise enough to burn this off, your blood sugar levels will go up, which will eventually lead to type 2 diabetes, fatty liver and the inability to lose weight.
How does this happen?
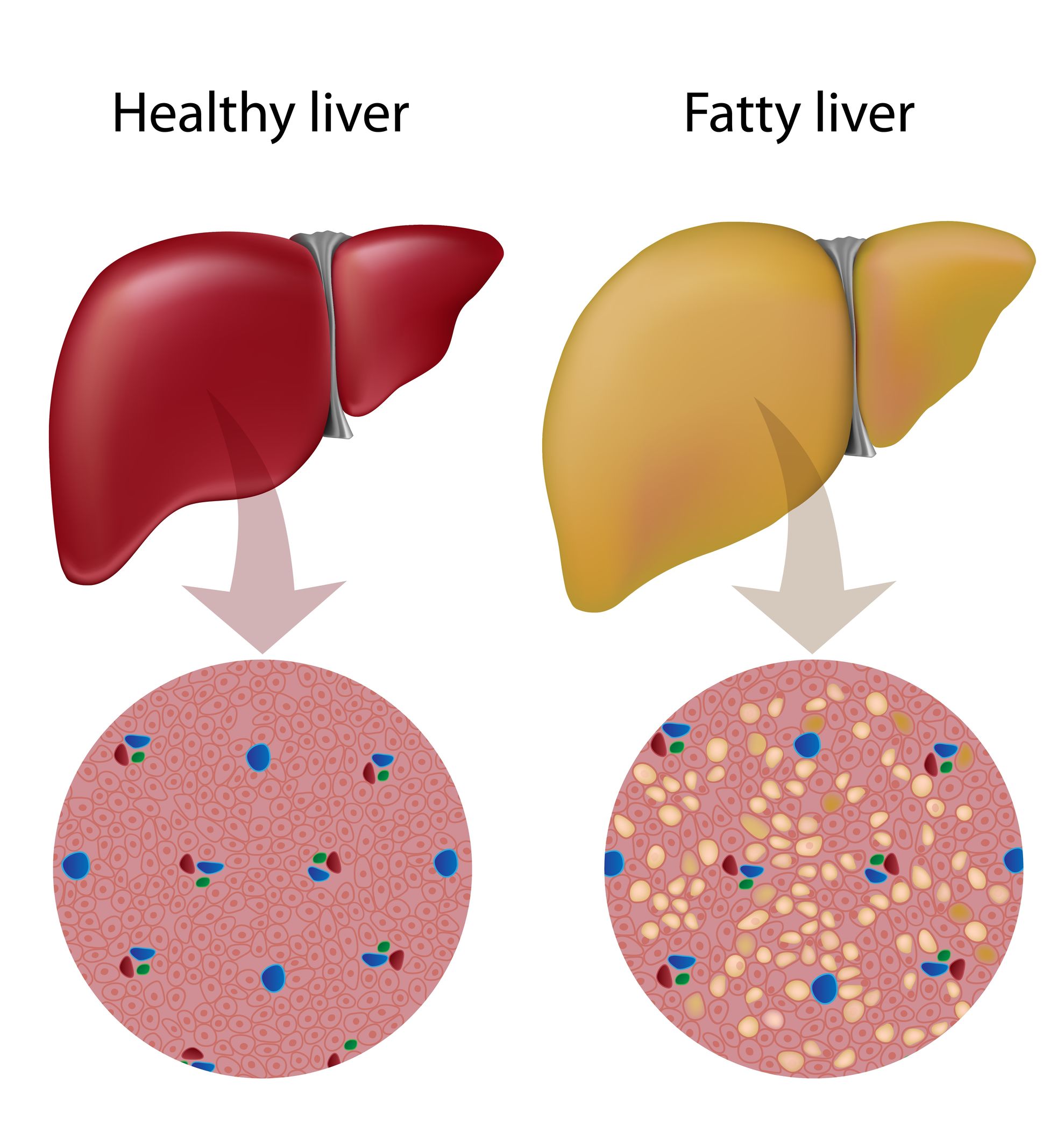
Your liver acts like a storage unit, so if there is too much sugar in the bloodstream with nowhere to go, the liver will suck it up and store it in the form of glycogen. Once the liver is full and there’s no more space for the excess sugar it will change or convert the sugar to cholesterol though a process that occurs both inside and outside the mitochondria of the cell, and subsequently spit it back out into the bloodstream to be stored somewhere else as fat.
Some of this fat can also be stored in the liver or around other organs, known as ‘organ fat’. Around the liver it is known as fatty liver and this serves as the short explanation.
For those of you who want to know how this works in a bit more detail you can read more here:
GLUCOSE METABOLISM: Glycation and Methylation
So, how does our bodies keep blood sugar under control?
There are generally 3 hormones that are directly responsible for blood sugar control:
INSULIN is produced in response to an increase in blood glucose levels and converts glucose to either glycogen in the muscles and liver (glyconeogenesis) or fat (lipogenesis).
According to Mike Julian your body should never have to rely on insulin alone to clear glucose from the blood. If you get to this stage then your are in trouble.
This is why exercise is so important.
GLUGAGON is produced in response to a drop in blood glucose levels and converts glycogen (stored glucose) back into glucose (glycogenolysis) or stimulate amino acid/protein conversion into glucose (gluconeogenesis).
This often occurs when there is stress, inflammation or sympathetic activation of the nervous system.
GLUCOCORTICOIDS are produced when blood sugar is low or when ACTH is released from the hypothalamus in response to stress or sympathetic activation.
They initially reduce inflammation, provide stress resistance, depress the immune system and increase blood glucose by breaking down protein (gluconeogenesis) and fat (lipolysis).
What Happens When Someone Follows a High Carbohydrate or Sugar Diet, or They Have High Blood Sugar Levels For Other Reasons?
The higher the blood glucose, the more insulin is secreted by the pancreas in an effort to open the GLUT receptors who’s job it is to allow glucose to enter the cells to be burned for energy inside the mitochondria. But when there is inflammation inside the cell as commonly seen in chronic disease or the CDR (Cell Danger Response), GLUT translocation to the cell surface will tend to be down-regulated. It means these little gates do not open as readily as we’d expect.
When the GLUT receptors fail to translocate or move to the cell membranes, or the insulin receptors become blunted/unresponsive we experience Insulin Resistance (IR). At this point it doesn’t matter how much insulin the body secretes, the cells simply do not respond.
There are two exceptions to this and it is the liver and the brain.
These two organs can absorb glucose into their cells without insulin or GLUT transporters as part of a survival mechanism.
But here is the real kicker…
INSULIN RESISTANCE IS PROTECTING YOUR CELLS
That’s right!
We often see Insulin Resistance (IR) as the problem and do our best to get insulin to respond, but maybe we should consider that this is an evolutionary protective mechanism to protect cells from an over-influx of glucose into the cells. It could be a real case of having too much glucose inside the cell due to a high GI meal, or as part of mitochondrial dysfunction where the mitochondria is unhealthy and cannot burn large amounts of glucose even within ‘normal’ limits.
After all, your body is not stupid. It doesn’t just do random things for the sake of it. It’s all in an effort to protect YOU.
Once glucose enters the cell and is converted into glucose-6-phosphate it cannot leave the cell. This is an irreversible conversion. In effect Insulin Resistance (IR) protects the cells from allowing any more glucose into the cells after glucose-6-phosphate levels are saturated.
Remember that the metabolism of glucose into energy/ATP produces a lot of free radicals in the process. If the body cannot cope with this it will do everything in its power to stop more free radical production as this will eventually lead to cell death. The body’s first priority is always survival. At this point it will slow down and alternative routes will have to be found to get rid of the excess glucose.
I talk more about this with Matt and Steve at ATP Science in the podcast on Fasted Cardio and Using Intermittent Fasting for Fat Loss which you can watch below:
LOW ENERGY PRODUCTION AFFECTS THE BINDING OF INSULIN
OK, so because of Insulin Resistance (IR) glucose is not able to enter into the cell. This keeps higher amounts of glucose in the bloodstream. High levels of glucose in the blood will keep stimulating insulin production without any effect, due to Insulin Resistance (IR). So now the liver has to make Insulin Binding Globulin (IBG) to bind all this excess insulin being secreted due to this Insulin Resistance (at least in the early stages before the pancreas becomes exhausted) which requires energy. If energy is being conserved as in the case of the CDR then liver function will suffer, less IBG will be produced, more unbound insulin will circulate causing blood sugar to drop and cause reactive hypoglycaemia.
Hypoglycemia is that feeling you get when you’ve had a meal, but shortly afterwards you feel hungry again, crave sugar, get the shakes, or feel jittery. You may even experience mood swings, irritability or anger.
GLUCONEOGENESIS RESPONDING TO REACTIVE HYPOGLYCEMIA
This is the process by which the liver will start to break down glycogen (stored glucose) or amino acids (protein and muscle) to produce glucose usually in response to low blood sugar levels.
This occurs when there isn’t enough glucose for energy production or when the body THINKS there isn’t enough glucose for energy production as in the reactive hypoglycaemia example above. In the latter case you can end up with something called glucotoxicity.
Reasons for a drop in blood sugar and gluconeogenesis signalling include:
- Endurance exercise (this will usually only happen in exercise lasting longer than an hour)
- Fasting
- Starvation
- Low carb/sugar diets
- Reactive hypoglycaemia
GLUCOTOXICITY
When very little insulin is secreted by the pancreas, even if blood sugar is high, the liver will interpret the low insulin secretion as a sign that there isn’t enough glucose for energy production and will start to make more through the upregulation of gluconeogenesis.
Not good!
It can really create a spike in blood sugar levels which will cause damage to the pancreatic β-cells and eventually lead to type 1 or insulin-dependant diabetes through the glycation process.
The excess glucose is then transported to the liver which activates fat synthesising enzymes in response to the higher concentrations of glucose. But it doesn’t just make any types of fats.
These fat synthesising enzymes will produce fatty acids such as palmitic and palmitoleic acid from glucose which is a very unhealthy type of fat. Free fatty acids combine to form triglycerides (3 fatty acids combined with glycerol). Some of this will be stored inside the liver cells and lead to fatty liver, and some of them will be transported back into the blood as free fatty acids and triglycerides.
I expand on this further down.
THE “DROWNING OF THE MITOCHONDRIA”
So, now you have lots of fuel – lots of glucose (high blood sugar) and lots of free fatty acids (liver making it from glucose).
All this fuel has to be burned for energy inside the mitochondria. This will put a tremendous nutritional strain on the mitochondria, and whatever does not make it down the oxidative phosphorylation pathway of the TCA (Tricarboxylic Acid or Krebs) cycle, will be shunted into the glycolytic pathway producing sorbitol (diabetes, cardiovascular disease, nerve damage), lactate (muscle pain, fibromyalgia, fatigue), purines (gout) and glyoxylation products (damaged proteins).
But that is not all.
The mitochondria is now working faster to try and burn of this excess fuel and in the process produce free radicals (ROS – reactive oxygen species). If these are not dealt with by a healthy intake of dietary antixodants (good diet, vegetables) or the body’s endogenous antioxidants (SOD, Glutathione for which you need healthy methylation) then they will cause further damage to blood vessel walls, cells and mitochondria which will slow this energy burning process down and make weight loss even harder.
This is why healthy mitochondria is so important in weight loss!
THIS IS HOW THE LIVER TURNS GLUCOSE INTO FAT
You can imagine the TCA cycle as being a spinning wheel.
By adding more fuel (glucose) you are spinning this wheel faster and faster (if the mitochondria is functioning well enough or able to) to produce energy/ATP. As the cycle spins all the while using up fuel, it spits out other intermediary products that’s needed elsewhere in the body.
Citrate is one of these intermediary products produced with every turn of the TCA cycle, and citrate is needed for fatty acid synthesis.
That’s right. Making more fats.
WHAT HAPPENS WHEN YOU TAKE STATINS
Statins is a common class of drug prescribed to those who have been diagnosed with high cholesterol levels and at risk for heart disease by the general medical profession.
 How do statins influence this whole process?
How do statins influence this whole process?
Statin drugs essentially stop the liver from making cholesterol and turning glucose into fat. This gives your liver no choice but to send all that excess glucose back into the bloodstream.
So, you may have great cholesterol levels, but your blood sugar will be higher, glycation will increase causing damage to the blood vessel walls and kidneys, and your risk for heart disease will keep on increasing (and now diabetes too) whilst you’re under the false impression that statins are helping you prevent all of this.
That is why it is very important that you monitor your blood sugar, insulin and HbA1c levels while on statins, as well as reducing your dietary carbohydrate and sugar intake.
STATINS – VITAMIN D AND COENZYME Q10
Cholesterol medications such as statins will also interfere with vitamin D production in the body as vitamin D is synthesized from cholesterol, as well as CoQ10 and steroidal hormones such as cortisol, estrogen and testosterone.
What are the first hormones to drop off as we age?
Estrogen in women and testosterone in men. The “youth hormones” in the different sexes.
What is the most commonly prescribed medication for the ageing population?
Statins.
Are we in fact accelerating the ageing process through these drugs? Yes.
Vitamin D is also responsible for immune regulation and has been linked to auto-immune disease and thus type 1 diabetes. The pancreas is also rich in vitamin D receptors (VDR’s). Vitamin D docks onto these VDR’s which then sends the message to the pancreatic β-cells to produce insulin. Insulin removes excess glucose out of the bloodstream. If vitamin D is deficient as a consequence of statin use, then these receptors won’t function properly, insulin is not produced and blood sugar spikes.
Vitamin D is also needed for muscle strength and growth, and the muscles determine the metabolic rate in the body. The more muscle you have the faster your metabolism (athletes) and the less muscle mass you have the slower your metabolism (obesity). This is because muscle cells contain lots and lots of mitochondria to produce energy for muscles to work hard. If muscles atrophy because of vitamin D deficiency and the mitochondria is sluggish because of CoQ10 deficiency, we’re going to end up with a lower metabolic rate, slower burning of carbohydrates, sugars and fat, and increases in blood sugar and weight gain.
In the absence of vitamin D, calcium is released from the bone into the blood to keep electrolytes balanced and blood pH under control. When calcium is higher than magnesium in the bloodstream it can lead to vascular constriction and increased blood pressure which raises the risk of cardiovascular disease.
Reduced heart mitochondrial function and heart muscle function due to CoQ10 deficiency may contribute even further to this.
We can see how statin drugs eventually leads to the exact opposite of its intended use.
STRESS INFLUENCES INSULIN
In acute stress (fight-or-flight) insulin levels will drop and blood glucose will increase. This is to allow more glucose in the bloodstream for quick energy production in order to ‘escape’ the dangerous situation and is a temporary survival mechanism that will revert back to normal when the stress is gone.
The longer this response continues for, the more sensitive the pancreatic β-cells will become to glucose. If the stress becomes chronic (longer than 30 days according to rat studies) then even smaller amounts of glucose will set off the release of large amounts of insulin as the pancreas becomes more responsive to glucose.
This could explain why some lose weight in the initial stages of stress, but eventually start to put weight on.
Increases in insulin will also increase glucose absorption into the cells as well as fat cells (adipocytes) and stimulate fat storage, at the same time slowing down the breakdown of existing fat stores in the body.
LIPOTOXICITY
Up until now we have discussed what happens when there is too much glucose for cells to absorb (glucotoxicity).
But what if there are too many fatty acids?
Fatty acids can come from a high fat diet or through increased fatty acid production as already explained. When the liver is exposed to higher concentrations of fatty acids it starts to produce more fat-carrying lipoproteins such as ApoB (Apolipoprotein B) which carries VLDL (Very Low Density Lipoproteins) and TG’s (Triglycerides) into the bloodstream. HDL (High Density Lipoproteins) and LDL (Low Density Lipoproteins) also become more saturated with these extra fats, and this is what contributes to cardiovascular disease and plaque formation in the blood vessel walls.
Notice how I say that HDL and LDL become saturated with fats. This is because HDL and LDL ARE NOT CHOLESTEROL. We talk about them as if they are cholesterol, calling them ‘good’ and ‘bad’ cholesterol, but they are not fats and NOT cholesterol. They are merely the carrier proteins for cholesterol.
Anyway, thought I wanted to point that out. Back to the conversation…
This however does not happen on a high fat LOW CARBOHDYRATE diet which is why it is very important to decrease carbohydrate intake when you increase your fats. If you start consuming healthy fats such as coconut oil, eating more bone broths, butter and ghee, and you’re still consuming your usual amounts of high glycemic carbohydrates, don’t be surprised if your blood glucose and lipid readings look worse next time you have them tested.
Don’t blame the fats. Blame the high glycemic carbohydrates.
References: