Not being a biochemist the numbers presented here may be slightly off (hopefully not too much).
The aim of the article is more to provide the bigger picture of the different cellular energy pathway and comparing their efficiency in providing the body with what it needs, how many resources they consume and reviewing which ones would serve your body best for future health benefits.
If this topic is new to you I suggest you first read Mitochondrial Dysfunction – What Does It Mean For You? to get a general overview of mitochondrial function and how it fits in with chronic disease.
Our cells contain organelles called mitochondria which is the energy-producing furnaces that metabolizes our food into electrons and eventually energy or ATP (adenosine triphosphate). Energy production can occur via two main pathways:
- Glycolysis
- Oxidative phosphorylation
Oxidative phosphorylation requires oxygen whereas the glycolysis pathway can produce energy in the absence of oxygen. This makes glycolysis an important ’emergency’ energy-production pathway when cells are under stress such as during injury, trauma or infections when oxygen levels may be compromised. However, oxidative phosphorylation is a far better way to produce energy.
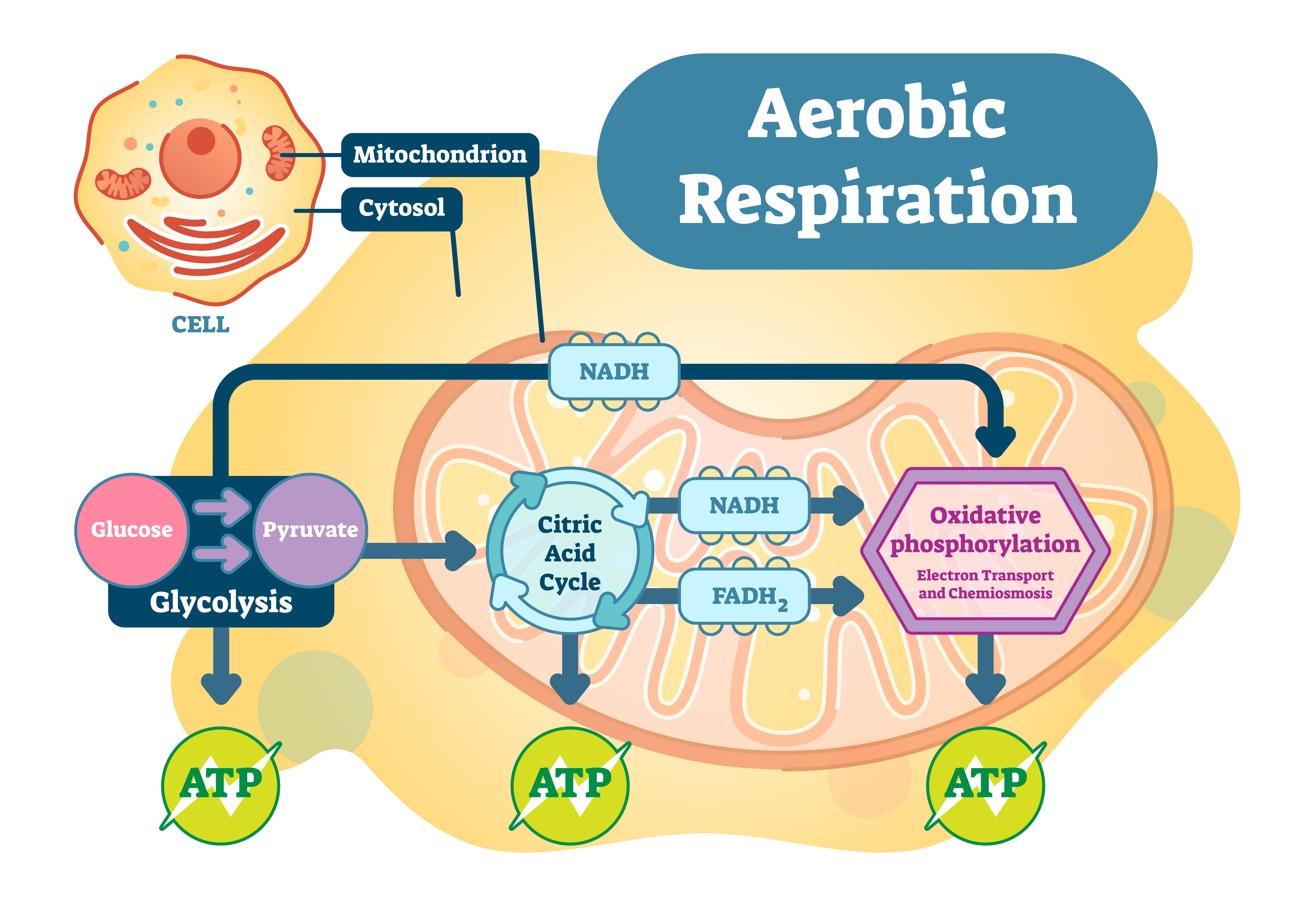
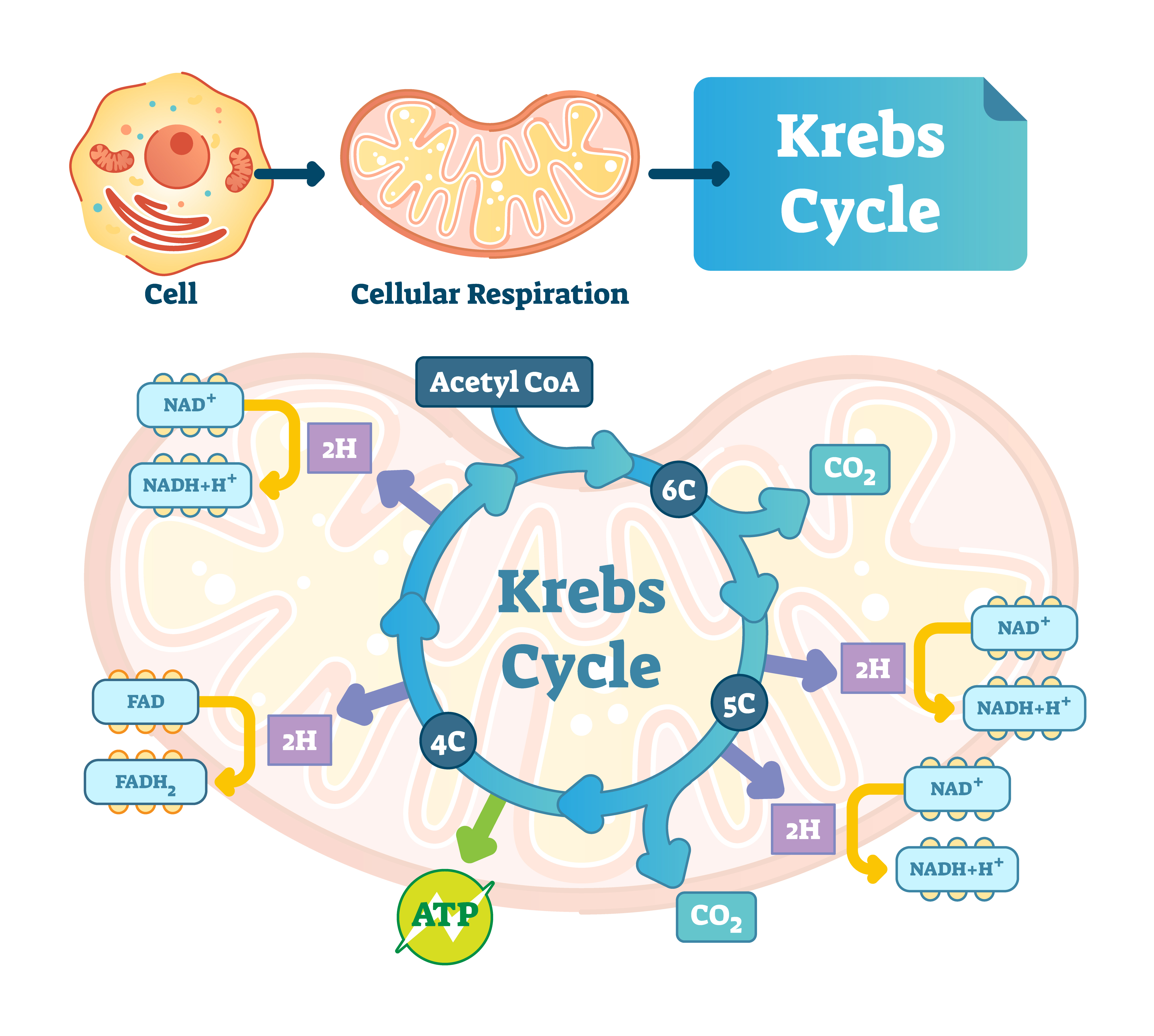
Energy Production using Oxidative Phosphorylation
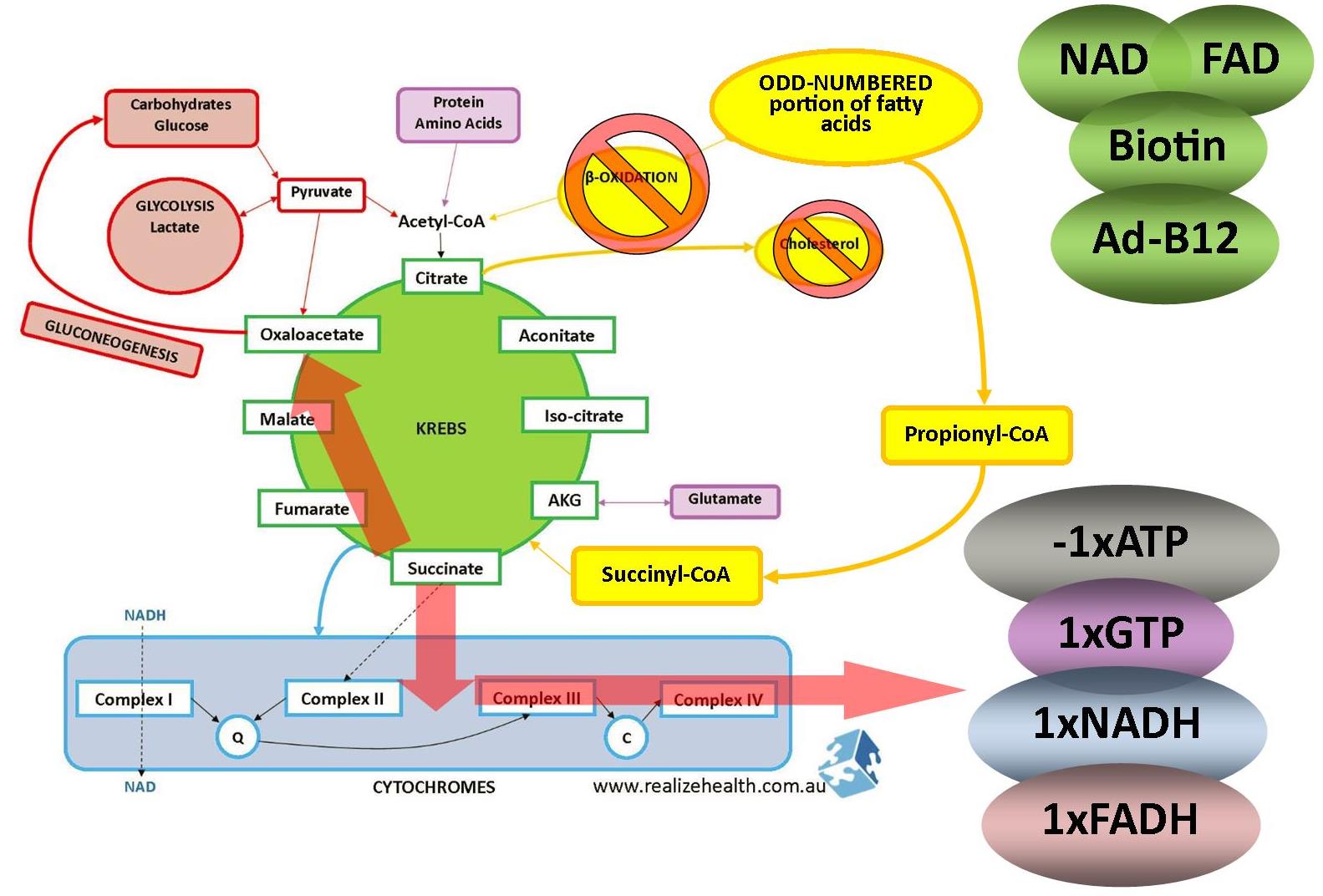
Oxidative phosphorylation requires oxygen and occurs within the krebs cycle, tricarboxylic acid cycle (TCA), or citric acid cycle as it is sometimes known. We will use the term krebs cycle for consistency.
The whole purpose of the Krebs Cycle is to take the food we eat, combine it with oxygen and convert it into cellular energy, carbon dioxide and water. This is a redox signalling molecular reaction where redox signalling molecules are produced, namely hydrogen, oxygen and chloride. Their functions are to:
- activate antioxidants needed to detoxify cells
- act as messenger molecules by regulating actions both inside and outside our cells
Hydrogen can combine with NAD (nicotinamide adenine dinucleotide) to form NADH and also released from NADH to form NAD again. The more food we eat, the more hydrogen is available to bind to NAD.
When this balance or homeostasis becomes disturbed we experience accelerated ageing and the development of chronic disease.
- Aged cells are cells that are still alive, but they’re not really doing their job at optimal capacity.
- Illness occurs when cells become dysfunctional and do things they shouldn’t be doing.
Cells are directed via redox reactions to heal themselves. They need to be in clusters so they can replace each other quickly as they die off, and they need to be stressed (to a healthy degree) to make them stronger and more resilient. Exercise and diet are the two things that influence redox reactions more than anything else.
Below is an overview of the krebs and electron transport chain (ETC – cytochrome) cycles and how food is processed:
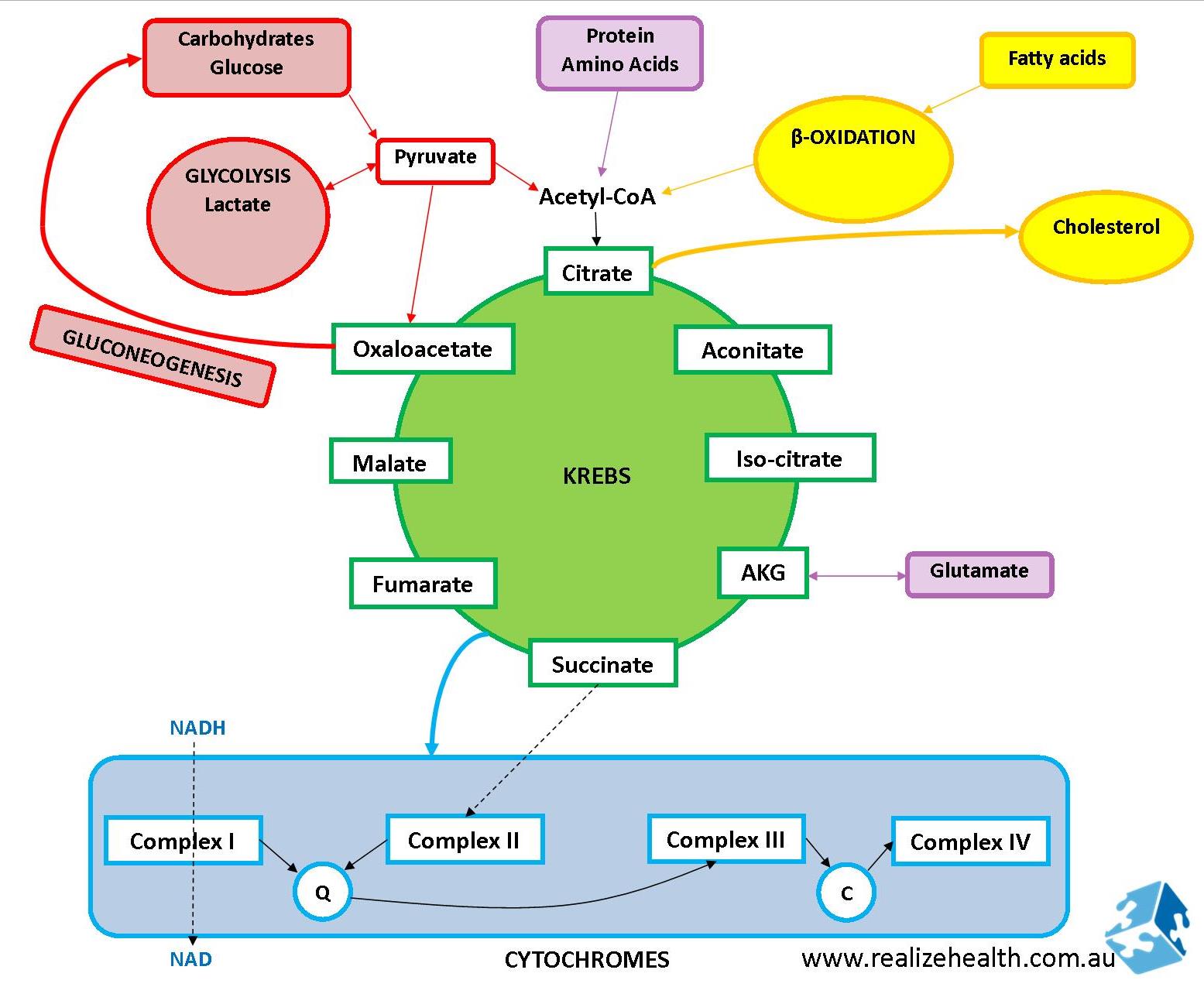
What is NADH?
NADH forms when hydrogen molecules bind to NAD. Hydrogen molecules come from food intake. NADH is thus a high energy molecule generated from food intake.
NADH enters the ETC and in the process produce free radicals. The more NADH is produced, the harder the mitochondria works, the more free radicals are produced. This is why alcohol and overeating reduces your lifespan and makes you age so quickly. NADH also signals the body that you’ve had enough to eat when it is high, so it may suppress hunger.
Fasting provides your body with the opportunity to clear the bottleneck of excess NADH trying to enter the ETC. The NAD/NADH ratio frequently fluctuates but is an important measure of how healthy the cells are. In mammals total ratio’s are anywhere between 3-10:1 for NAD:NADH. When the ratio is more in favour of NADH there will be increased free radical production (such as hydrogen peroxide) and likelihood of cellular damage. On the other hand when the ratio favours NAD (as in caloric restriction) it acts as a sensor that regulates and activates genes related to extended lifespans such as the SIRT genes.
When Are We Likely To Have High NADH
- Overeating
- High sugar/carb diet
- Alcohol
- Candida infections
- Low oxygen states
- Upregulated glycolysis
When Are We Likely To Have Low NADH
- Prolonged fasting or starvation
Time-Restricted Eating and Chronic Disease
When Are We Likely To Have Low NAD
- Diets deficient in B vitamins
- Infection (viruses such as Haemophilus influenzae cannot make their own NAD so they use yours)
- Gut dysbiosis (candida cannot make their own NAD so they use yours)
- Ageing
Energy Production using Glycolysis
As mentioned before the glycolytic cycle is not a very efficient energy-production pathway, but it does provide quick energy when needed in emergency situations.
Some cells are devoid of mitochondria and rely solely on glycolysis for survival such as red blood cells, cancer and stem cells. For each glucose molecule that is broken down 2 ATP and 2 NADH molecules are produced. Apart from in the cells mentioned, this pathway predominates in the following events:
- Yeast/candida infections
- Cancer
- Low oxygen states
- Blocks to the krebs cycle
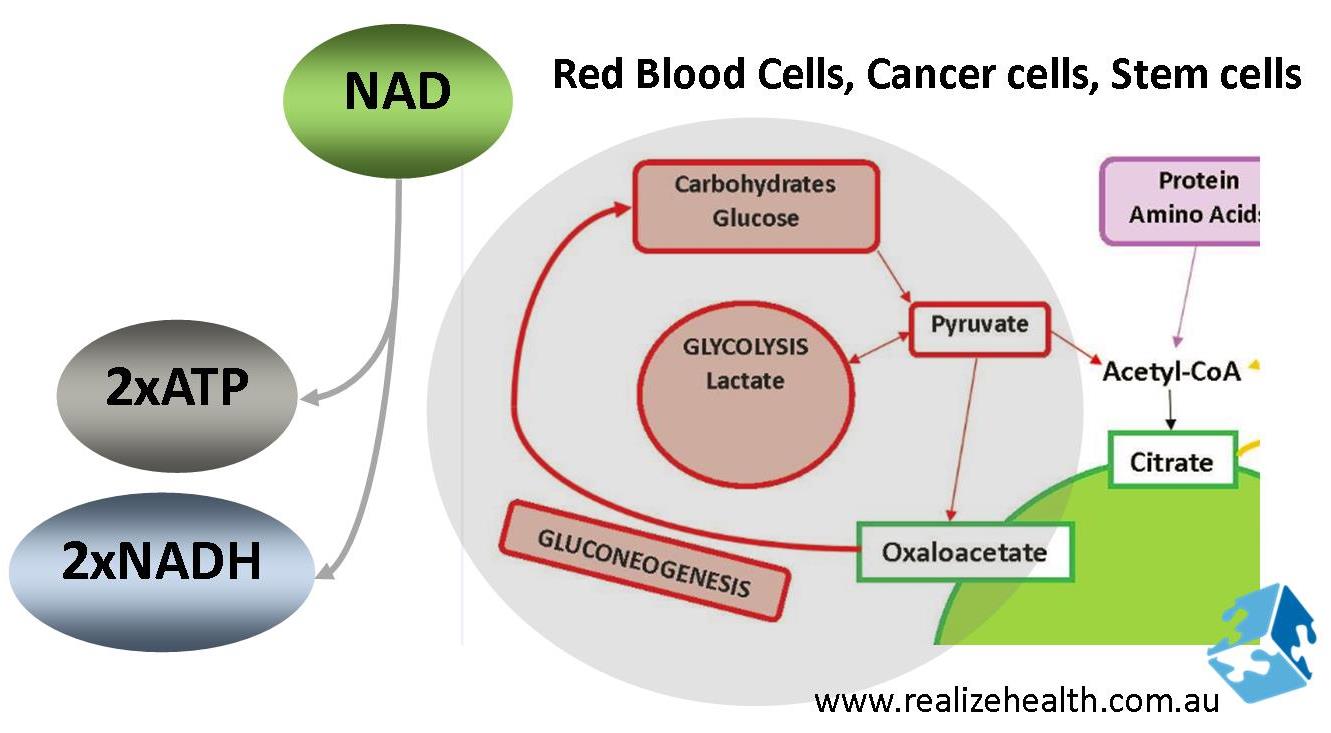
Note that the NADH production doesn’t seem that high, but if we take the ratio’s of ATP and NADH produced they sort of cancel each other out, meaning there isn’t really any extra ATP to make everything else work better or more efficiently. The glycolytic pathway is also the primary pathway for NADH production because it’s so quick.
During emergencies where cells are damaged due to mechanical trauma or infections, this pathway becomes up-regulated in order to shift energy production away from oxidative phosphorylation in a bid to slow down the ETC, preserve oxygen and reduce reactive oxygen species (ROS) formation as a way to protect the cell against further damage. If more NADH is produced than is needed because we eat more than we should, then energy-production will continue down these alternate glycolytic pathways and produce ROS (free radicals) in the process which will bring us right back to square one, creating cellular stress and eventual cell death.
FAT METABOLISM: The Basics
Fats are made up of fatty acid chains – some short, some long – some with even-numbers, some with odd-numbers. To put this into perspective, this is what we mean:
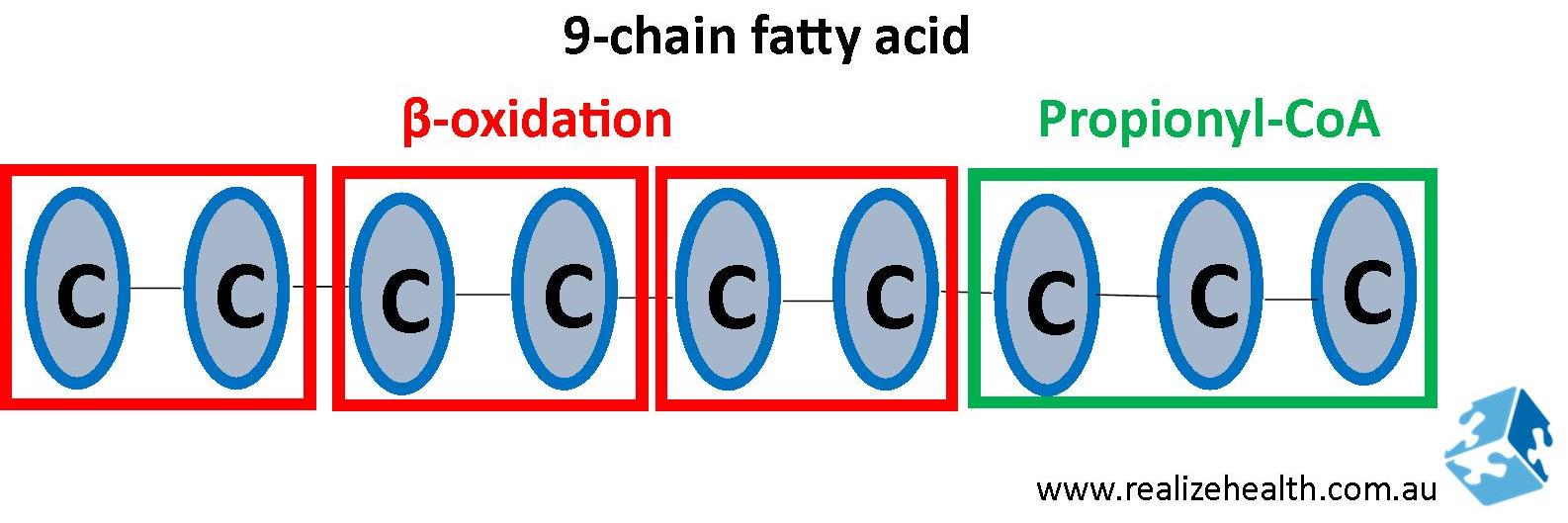
During fat metabolism the even-numbered single bond fatty acids will be metabolized for energy through β-oxidation and broken up into acetyl-coA. The odd-numbered chain will enter the krebs cycle via propionyl-CoA (explained further below).
Fatty acids can be classified into short-, medium- or long-chain fatty acids based on the number of chains.
- Short chain fatty acids = 2-6 chains
- Medium chain fatty acids = 8-10 chains
- Long chain fatty acids = 12-24 chains
Fat Metabolism: NADH and β-oxidation
Those following ketogenic diets may be more familiar with the term β-oxidation. Fats broken down via β-oxidation produce ketones in the process.
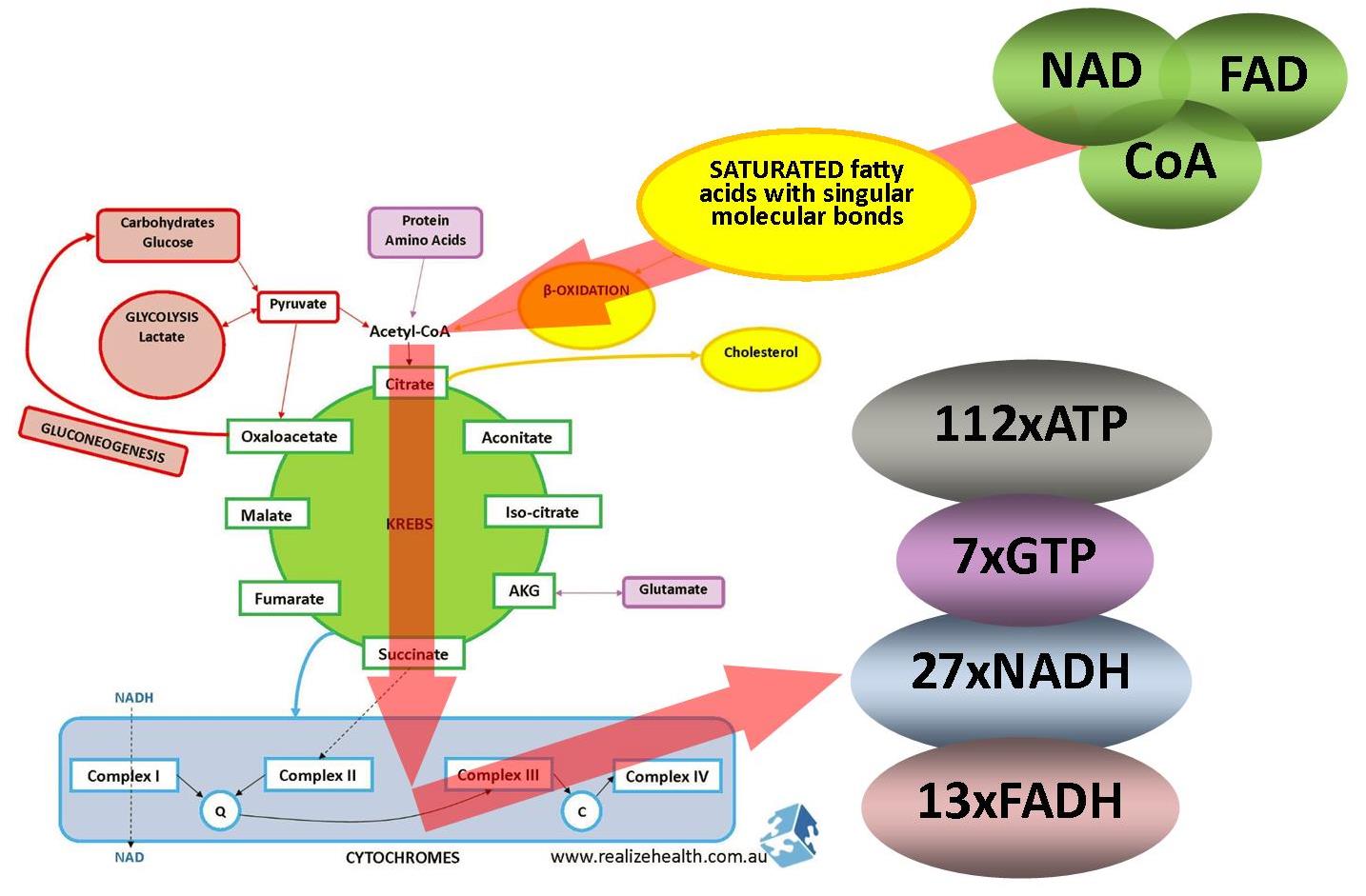
The process of β-oxidation produces a tremendous amount of energy both in the form of ATP and GTP (guanosine triphosphate). GTP plays an important role in the arginine pathway responsible for nitric oxide (NO) synthesis and thus vasodilation and cardiovascular health. But it also uses up a lot of co-factors such as vitamin B2 (riboflavin), B3 (niacin) and acetyl-carnitine, and requires healthy thyroid function.
To a large extend it is a self-supportive pathway as the co-factors are regenerated in the electron transport chain (ETC) ready for another cycle. The rate-limiting step would be the amount of acetyl CoA available.
It is important to note that β-oxidation inhibits glucose oxidation. This means that when a diet is high in both fat and sugar, glucose will both accumulate in the blood vessels increasing blood glucose levels and go through glycolysis forming sorbitol and other harmful by-products that contribute to chronic disease. It will certainly not be broken down for energy.
On the other hand, if carbohydrates are severely restricted as in a ketogenic diet, fasting or fasted exercise, ketones will be formed which do not require CoA (coenzyme A) to enter the mitochondria. This continues fat burning for a longer period of time as the state of ketosis is maintained.
Interestingly enough it is not as easy for the body to break down unsaturated fatty acids. Unsaturated fatty acids have double bonds which need additional enzymes to break the bonds before they can be allowed to enter β-oxidation. These enzymes can only shift the double bond position on naturally occurring unsaturated fatty acids with cis-configurations and can’t do so on trans-configurations.
Hydrogenated unsaturated fatty acids contain both cis and trans configurations and will only be partially metabolised. Certainly another good reason to stay away from hydrogenated fats found in many processed foods.
You will also notice that more NADH is produced than in glycolysis, but looking at the ratio’s between ATP and NADH, the amount of ATP produced far outweighs NADH production. More ATP/energy available will put less strain on the mitochondria and other organ systems.
Fat Metabolism: Odd-Numbered Fatty Acids
These are found in plants and marine organisms. For the purpose of this explanation we’re only looking at the 0dd-numbered portion of fatty acids in a long chain of fatty acids. Keep in mind that a 19-carbon fatty acid chain is an odd-numbered fatty acid but most of this will go through β-oxidation as explained above (2-carbon fatty acids with a single bond between them).
Because it doesn’t enter β-oxidation and expel citrate, it’s not really involved in cholesterol synthesis. It enters the krebs cycle as propionyl-CoA and succinyl-CoA where it either contributes to energy production or move towards oxaloacetate if amino acids or glucose is needed.
Alcohol Metabolism and NADH
This deserved a post of its own and you can read about it here:
What happens when you drink alcohol?
The Common Link Between Candida, Alcohol and Multiple Chemical Sensitivities
What is NADP?
Nicotinamide adenine dinucleotide phosphate is a cofactor in anabolic or building reactions such as making cholesterol and nucleic acids for DNA and RNA. It becomes important when the body needs to repair itself but can also be in high demand during infections and cardiovascular disease.
Pathogenic organisms such as viruses and bacteria can manipulate the host’s biochemistry and upregulate pathways such as this to make more building blocks for themselves so they can grow and multiply.
In the case of cardiovascular disease cholesterol is often produced in excess to repair damage to the blood vessels. Think of cholesterol as the ‘cement’ to fix the ‘pot holes’. Excessive damage to the blood vessels caused by free radicals from eating too much or not consuming enough antioxidant-rich foods such as vegetables may increase the need for NADP and upregulate these building-reactions.
What is NADPH?
NADPH is the reduced form of NADP and is formed via the pentose phosphate pathway (PPP) in glycolytic metabolism. It allows glutathione to be regenerated and is very important for antioxidant status. Being a reductive agent it is anabolic in nature. Other functions of NADPH include:
- Cholesterol synthesis
- Generating free radicals (superoxide and hydrogen peroxide) in immune cells to fight pathogens
Superoxide in turn combines with nitric oxide to form peroxynitrite which is highly damaging to cells. The glutathione produced and recycled using NADPH as a cofactor keeps this in check.
Fat Synthesis: NADPH and Making Cholesterol
Cholesterol is made outside of the mitochondria in the cytosol. Mitochondrial intermediaries cannot cross the mitochondrial membrane with the exception being citrate. Acetyl-CoA and oxaloacetate combines to form citrate inside the mitochondria. Citrate then crosses the mitochondrial membrane to then again split up into oxaloacetate and acetyl-CoA. Oxaloacetate enters back into the pyruvate cycle for energy production and produce NADPH in the process. That NADPH is then used again to assemble acetyl-coA back into fatty acids and cholesterol.
This is an alternative pathway to the PPP for NADPH production.
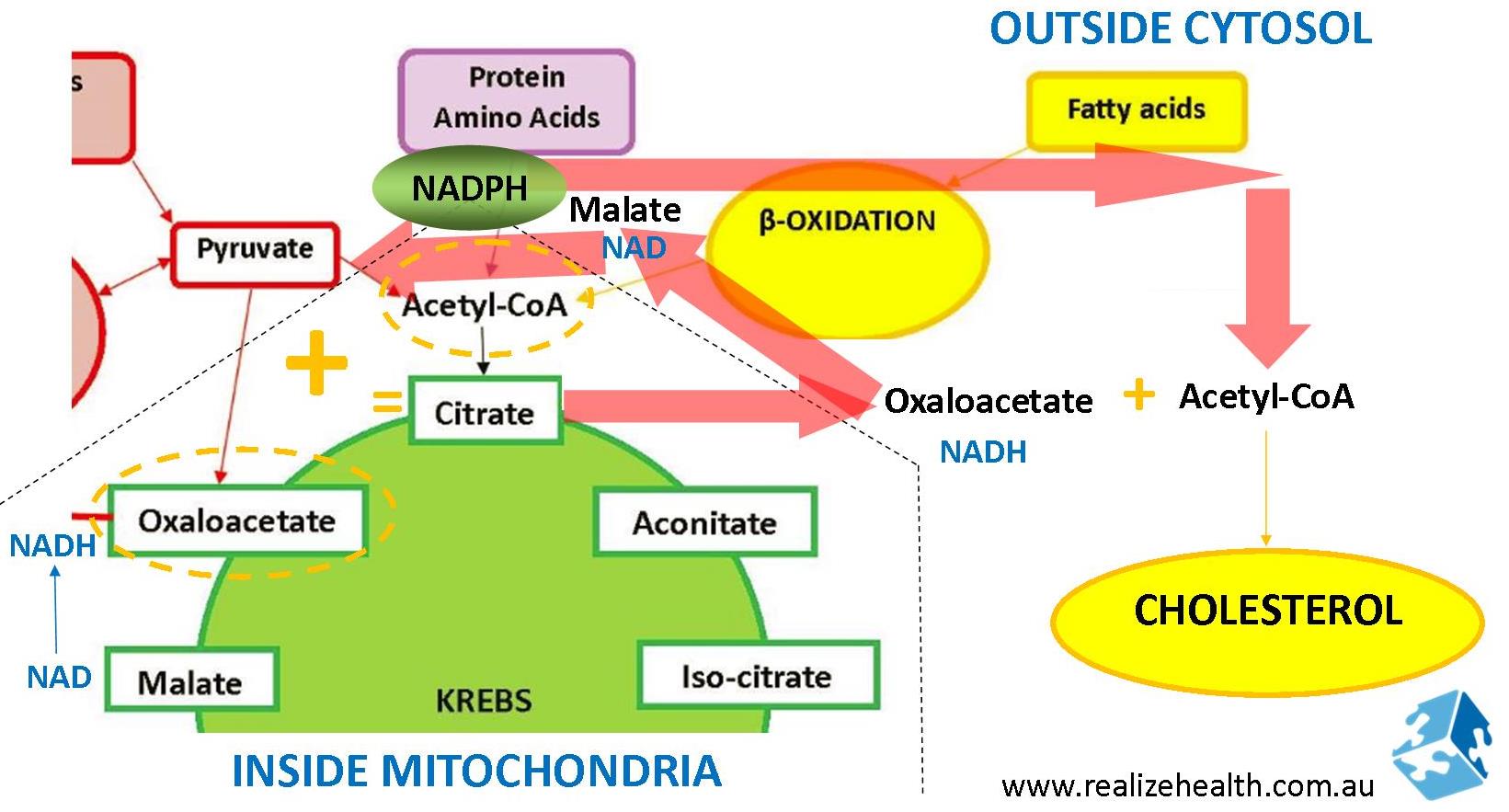
Remember that excessive NADH will inhibit acetyl coA entry into the mitochondria which will also increase cytosol acetyl coA levels. This is one of the mechanisms how alcohol and overeating increase cholesterol production. By increasing NADH levels, preventing food to be metabolized into energy, and shifting it towards making cholesterol instead. This is typically what happens with cardiovascular disease and metabolic syndrome (fatty liver).
Metabolic Syndrome and Fatty Liver
Atherosclerosis
NADPH is needed to regenerate glutathione and glutathione as an antioxidant needs to protect and repair blood vessel walls. If the pathway favours cholesterol synthesis because of alcohol, overeating, gene down-regulations or nutritional deficiencies, then it doesn’t leave much NADPH available for glutathione regeneration. Not only are you producing more fatty acids and cholesterol, but also providing them with opportunities to attach to blood vessel walls.
Atherosclerosis and the Biochemistry Behind It
References: