Could A Sensitivity To Phenols Be The Reason For Your Symptoms?
In our practice we see many clients with phenol sensitivities, or symptoms that reflect phenol sensitivities.
In this article we explore what they are, some of the symptoms they may be responsible for, and what we can do to minimize these symptoms while treating the root cause responsible.
But first…
How do you know that you may have a problem with phenols?
Being more sensitive to foods containing salicylates, colourful fruits and vegetables, spices, herbs (even medicinal herbs) can be an indication. This is problematic because it includes many of the healthy foods that we need for fiber, antioxidants, vitamins and minerals.
Parents will often note that rashes or behaviours get worse when their child has consumed anything with a lot of colour such as cordials. This can sometimes be mistaken for sugar-reactions when it is more due to the high phenol content.
Other things that may indicate a problem with processing phenolic compounds include being more sensitive to chemicals, suffering with infections or gut dysbiosis, and getting worse every time you try and treat them.
Typical symptoms of phenol or salicylate sensitivity include:
 Emotional extremes
Emotional extremes- Inappropriate laughter
- Dark circles under the eyes
- Red face/ears
- Allergy-type symptoms such as hives, asthma
- Chronic infections such as ear infections, sinus problems
- Diarrhea
- Headaches
- Sensitivity to noise, light, touch
- Trouble falling asleep at night, waking up during the night, insomnia
- Fatigue
- Irritability
- Night sweats
- Bed wetting and day wetting
- Aggression esp. when exposed to petroleum-based phenols
- Self-injury such as head banging, hitting
- Hyperactivity in children
- Learning difficulties such as dyslexia, speech difficulties
- Chronic fatigue in adults
So what are these compounds that can create so much havoc in your or your child’s body exactly?
WHAT ARE PHENOLS?
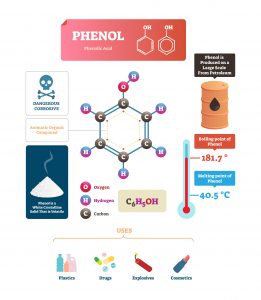
Phenols are compounds made up of a 6-carbon benzene ring with one hydroxyl (-OH) group bonded to it. Phenols will have other groups attached as well depending on the type of phenol.
They are mainly secreted by plants, animals or microbes to protect themselves against invaders, toxins, pesticides and inhibitors. Phenols are similar (but not the same) to alcohols from a biochemistry standpoint which makes them more toxic or reactive than benzene.
CHEMICAL PHENOLS
Probably the most well-known chemical phenols would be the bisphenol A (BPA) found in plastics, but also toluene, petroleum, benzene, coal tar and xylenol.
Many chemical phenolic compounds are found in disinfectants, antiseptics, food additives, detergents, fungicides (used for waxing citrus fruits), and medications such as aspirin. From these chemical phenols benzene is more toxic than toluene. The reason for this seems to be in the bond strength according to William E Donges. Because toluene has weaker bonds it is more easily metabolized by the liver P-450 cytochrome system. Benzene is slower to be oxidized and can thus take longer to clear out of the system, allowing it more opportunities to damage DNA.
Reversing Damage to DNA in Chronic Illness and Fertility
ENDOGENOUS PHENOLS
These include catechols or catecholamine-like compounds such as dopamine, norepinheprine, epinephrine (adrenalin) and estrogen.
Strictly speaking they are not phenols as phenols only contain one hydroxyl group, whereas catechols contain two hydroxyl groups, but structurally they are very similar. Once an amine group is attached to the catechol, such as during methylation via COMT, it is called a catecholamine.
Because phenolic compounds from different sources are so similar in chemicals structure, different phenols can act to disrupt these hormones and neurotransmitters. A good example is the phenolic compound, 4-cresol, produced by clostridia which is a known dopamine disruptor. This can be measure with an Organic Acid Test.
DIETARY PHENOLS
Polyphenols, flavonoids, resveratrol and salicylates are naturally occurring phenols produced by plants, and is found in fruits and vegetables. When plants are stressed through drought or insect attacks they will produce more of these polyphenolic compounds (resveratrol being the better-known one) which is associated with better antioxidant protection from cancer and chronic disease. These phenols are usually methylated or esterified and are conjugated which means they are found in the glycoside form and the aglycone form.
Some food additives and preservatives will also contain phenols, but this would fall more into the category of chemical phenols.
Dr. Benjamin Feingold (pediatric allergist) is well-known for his research into links between high phenol-foods, allergies and behavioural problems in children. Both the Feingold Diet and FAILSAFE Diet can be used to eliminate phenols and salicylates and you can also look at our PHENOLS Food List. Failsafe eliminates more food chemicals than Feingold so it may be easier to start with something like the Feingold diet as this may be all that you need. However, if you suffer from chemical sensitivities you may have to upgrade to Failsafe.
BACTERIAL PHENOLS
Bacteria and other organisms produce phenols in response to some kind of stress, insect attacks, pathogen attacks, being wounded, etc. so it would make sense that when you are taking antimicrobials for infections you will have more phenols released into your system which can become a problem if you can’t clear them properly. This is most likely why many people may feel worse when they start taking antimicrobial protocols.
4-Cresol produced by clostridia is another common phenol that has the ability to disrupt enzyme function such as DBH (Dopamine Beta Hydroxylase) which we mentioned further up.
But it’s not all bad.
Benzoate or benzoic acid itself is a phenol-compound that has an antimicrobial effect which is why it is used as a preservative in many foods. So, when bacteria break down polyphenolic compounds found in many herbs and plants they produce this antimicrobial agent that helps to kill off unfavourable organisms.
This is interesting.
A good example is cranberries which are high in polyphenolic compounds. Cranberry extracts have been used in the treatment of urinary tract infections, and even periodontitis, with many studies proving its effectiveness in these conditions. This is one of the major reasons why fruit and vegetables are so important to keep us healthy. In fact, benzoic acid has strong anti-fungal properties against candida albicans.
Issues arise when there is an overgrowth of bacteria producing too much of their own phenolic compounds, and at the same time stimulating the conversion of dietary polyphenols into benzoic acid which then interferes with mitochondrial function and puts pressure on phase 2 liver detoxification as well as the PST (Phenol-Sulfur-Transferase) enzyme.
SOIL PHENOLS
Large amounts of phenols are released from decomposing plants which are further transformed and oxidized by soil microbes into fulvic and humic acids. These acids bind to clay minerals and metal hydroxides and have many health benefits.
METABOLISM OF PHENOLS
After ingesting phenols from foods it goes through xenobiotic metabolism which involves CYP2E1 and CYP3A4, but I’m sure there’s other CYP enzymes involved here as well. Phenols are then conjugated in phase 2 liver metabolism using glutathione, sulfur (SULT1A), glycine, phenol-sulfur-transferase (PST) or glucuronic acid. The latter depends on the gene UGT1A6 which encodes for UDP glucuronosyltransferase which has major activity on phenols such as coumarins, anthraquinones and flavones. Metabolism of phenols occurs through aryl-alcohol dehydrogenase which uses NAD (vitamin B3) to produce an aromatic aldehyde.
Phenols are rapidly absorbed through the skin, lungs and gastrointestinal tract. Once it is conjugated or bound to something, it can stay in the body for 1-5 hours. So what are the most common routes of elimination?
- Glucuronidation through forming phenyl glucuronide
- Sulfation through forming phenyl sulphate
Both PST and SULT1A enzymes need PAPS (3-phosphoadenosine-5-phosphosulfate) to function properly. Once phenols increase and start to overwhelm PAPS, metabolism switches more towards the pathway of glucuronidation.
Phenols are drawn to fats (lipophilic), denatures proteins and disrupt disulphide bridges in keratin on the skin. In severe cases it depresses the CNS. This is why we see neurological problems such as hyperactivity, aggression, learning difficulties and other behavioural issues in children with phenol sensitivities.
Phenols are formed via 3 different pathways:
- Shikimate/chorizmate or Succinylbenzoate pathway –> producing phenyl propanoid derivatives
- Acetate/malonate or Polyketide pathway –> produces the side-chain-elongated phenyl propanoids, including flavonoids and some quinones.
- Acetate/mevalonate pathway –> produces aromatic terpenoids (monoterpenes) by dehydrogenation reactions.
ARYLOXIDES
If a phenol looses a hydrogen group (H) of the hydroxyl group (-OH), it forms a negative phenolate or phenoxide ion which are called aryloxides. Phenoxides can conjugate to other minerals such as sodium to form sodium phenoxide. Because of this loss of a H+ ion phenols are more acidic than water.
IN SUMMARY…
Phenols are important compounds found in nature that has a role in keeping us and our microbiome healthy.
Issues arise when our phenol detoxification systems becomes overwhelmed with too many phenols from environmental chemicals, bacterial toxins, processed foods containing high amounts of phenols, etc.
If you add in enzyme inhibitions such as PST from SIBO (Small Intestinal Bacterial Overgrowth) or nutritional deficiencies it amplifies all of the above and we end up with phenol sensitivities.
Phenols are not bad but can be responsible for many of the discomforting symptoms you may be experiencing. Eliminating them from the diet though is a short-term solution. Working towards clearing the pathways and dealing with infections should be the focus for long-term resolution.
Good resources to look at include:
References:
https://fscimage.fishersci.com/msds/02610.htm
http://avogadro.chem.iastate.edu/MSDS/phenol.htm






Hi, you said that benzoic acid is a phenolic compound. But the wikipedia phenols page says that it is a phenolic compound if it’s an aromatic hydrocarbon ring with a hydroxyl _OH attatched to the ring. Am I mistaken here? I would happy to hear from you and get enlightened.
Thank you
https://en.m.wikipedia.org/wiki/Phenols
Benzoic acid structure formula doesn’t seem to have hydroxyl group attached to it. So, should we consider it a non-phenolic compound?
Strictly speaking yes. But your gut bacteria will turn it into a phenolic compound.
Hi Hame,
That’s a good question. I’ll first state that I’m not a biochemist even though biochemistry was one of my subjects. I tend to look at things from a ‘big picture’ lense and try not to get too caught up in the details.
However, in answering your question, you are correct that benzoic acid is a carboxylic acid due to the attached hydroxyl_OH ring. Phenolic acids include both substances with a phenolic ring and an organic carboxylic acid ring. Benzoic acid is also responsible for producing the phenolic acids hydroxybenzoic and hydroxycinnamic acids. So we could argue that benzoic acid is not a phenolic acid, but it does produce phenolic acids as an end-product. It really depends how specific you want to get.
If you want to be correct in your terminology then benzoic acid is a non-phenolic compound that produces phenolic compounds in the gut.
Hope that helps :).